Week2
MANUSCRIPT ID
- Title
Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders
- Reference
Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, Soma M, Takao K, Tanda K, Ohira K, Toyama K, Kanzaki K, Fukunaga K, Sudo Y, Ichinose H, Ikeda M, Iwata N, Ozaki N, Suzuki H, Higuchi M, Suhara T, Yuasa S, Miyakawa T.Mol Brain. 2008 Sep 10;1(1):6. [1]
- Abstract
Elucidating the neural and genetic factors underlying psychiatric illness is hampered by current methods of clinical diagnosis. The identification and investigation of clinical endophenotypes may be one solution, but represents a considerable challenge in human subjects. Here we report that mice heterozygous for a null mutation of the alpha-isoform of calcium/calmodulin-dependent protein kinase II (alpha-CaMKII+/-) have profoundly dysregulated behaviours and impaired neuronal development in the dentate gyrus (DG). The behavioral abnormalities include a severe working memory deficit and an exaggerated infradian rhythm, which are similar to symptoms seen in schizophrenia, bipolar mood disorder and other psychiatric disorders. Transcriptome analysis of the hippocampus of these mutants revealed that the expression levels of more than 2000 genes were significantly changed. Strikingly, among the 20 most downregulated genes, 5 had highly selective expression in the DG. Whereas BrdU incorporated cells in the mutant mouse DG was increased by more than 50 percent, the number of mature neurons in the DG was dramatically decreased. Morphological and physiological features of the DG neurons in the mutants were strikingly similar to those of immature DG neurons in normal rodents. Moreover, c-Fos expression in the DG after electric footshock was almost completely and selectively abolished in the mutants. Statistical clustering of human post-mortem brains using 10 genes differentially-expressed in the mutant mice were used to classify individuals into two clusters, one of which contained 16 of 18 schizophrenic patients. Nearly half of the differentially-expressed probes in the schizophrenia- enriched cluster encoded genes that are involved in neurogenesis or in neuronal migration/ maturation, including calbindin, a marker for mature DG neurons. Based on these results, we propose that an "immature DG" in adulthood might induce alterations in behavior and serve as a promising candidate endophenotype of schizophrenia and other human psychiatric disorders.
- Keywords
alpha-CaMKII, working memory, dentate gyrus, schizophrenia, human post-mortem study, endophenotype
- Input Author
SMW
MANUSCRIPT DETAILS
- Introduction/Aims
A previous study showed forebrain-specific calcineurin knockouts had abnormal behaviors associated with schizophrenia and impaired working memory.
In this study, the group made knockouts for seven genes encoding proteins potentially involved in calcineurin signaling.
Only the alpha-CaMKII null mutation heterozygotes (alpha-CaMKII+/-) had working memory deficits and were discussed in this study.
CaMKII is a ubiquitous serine/threonine protein kinase that is abundant in the brain (up to 2% of the total protein). It is a holoenzyme that consists of four isozymes (α, β, γ, δ), and it phosphorylates protein substrates, such as AMPA receptors, synapsin I, tyrosine hydroxylase, L-type Ca2+ channels, MAP-2, and itself by autophosphorylation. CaMKII is important for long-term potentiation, synaptic plasticity, and memory formation, and it may be downstream of calcineurin according to a model.
This paper aims to describe a potential endophenotype, which they call "immature dentate gyrus," which was seen in the CaMKII heterozygotes and which may serve as a predictor for schizophrenia and/or bipolar disorder.
- Methods
(Note: this section only covers methods for which the data was shown in the paper. Several more experiments were done but the results were shown in supplementary material available at [2])
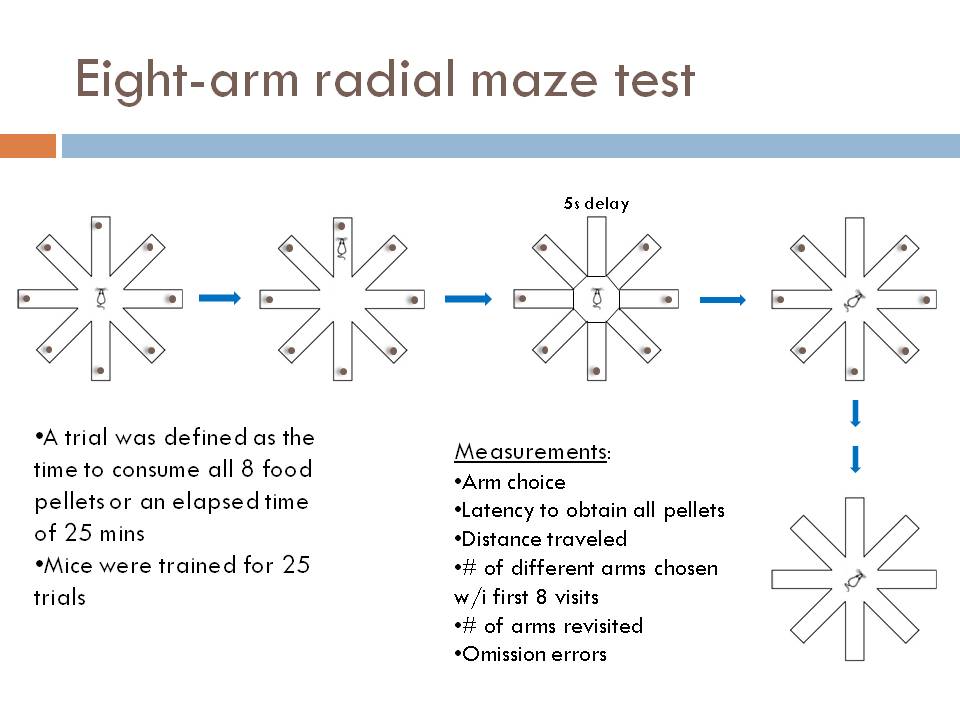
1) Working memory eight-arm radial maze test Each arm of an eight-arm radial maze was loaded with food, and the mice were allowed to go into each arm of the maze to obtain the food, with a 5 second waiting period after each time they re-entered the center of the maze. This tests working memory by testing whether the animal remembered which arms it had already visited during the trial. The food was not re-loaded during the test, and a test ended either when the animal had retrieved all of the food, or in 25 minutes, whichever occurred first.
2)Reference memory eight-arm radial maze test Only one of the eight arms was consistently loaded with food and the animals were allowed to enter the arm of the maze to obtain the food. This tests reference memory because it tests whether the animal learned which arm always contained food.
3) Locomotor activity measurement The locomotor activity of the mice in their home cage was measured automatically with a camera which recorded the location of the mouse at one frame per second. Activity was recorded during the animals dark (active) period for at least 3 months.
4) Microarray analysis was conducted using standard methods and a Affymetrix mouse genome array. The results were validated with quantitative real time PCR.
5) Dopamine D1 receptor binding and NMDA receptor binding was tested using probes.
6) For a few of the genes identified on the microarray, ene expression levels in different brain regions were compared according to the Allen Brain Atlas ([3].
7) C-fos expression after foot shock was compared between mutant and control mice.
8) Brd-U labeling was used to label new cells in the dentate gyri of mutant and control mice.
9) Cells in the dentate gyri of the mutant and control mice were labeled for calbindin (a mature neuron marker), PSA-NCAM (a late progenitor and immature neuron marker), and for calretnin (an immature neuron marker).
10) Rapid golgi staining was used to show the morphology of the cells in the dentate gyri of mutant and control mice.
11) Electrophysiological recordings were made using the cell patch clamp technique.
12) Post-mortem human tissue A) Gene expression of 166 human brains was measured using microarray analysis, and the brains were then clustered according to differential expression of 10 genes that were differentially expressed in mutants. B) The gene expression of controls from the "control" cluster and 19 disordered patients from the "disordered" cluster was compared.
- Results
1) In the spatial working memory version of the eight-arm radial maze, the mutants did worse than control mice with respect to the number of different arm choices in the first eight entries (P = 0.0022) and the number of revisiting errors (P = 0.0026).
2) Mutant mice had normal performance in reference memory tasks in the eight-arm radial maze (P = 0.6394).
3) Mutant mice were more hyperactive than control mice, had a more variable activity pattern (coefficient of variation, P = 0.0088, Mann-Whitney U test), and the activity of mutant mice increased throughout the dark period (genotype effect P < 0.0001, interaction between genotype and time P < 0.0001, repeated measures ANOVA).
4) More than 2000 genes were differentially expressed in mutants compared to controls, including a two fold increase in dopamine D1 receptor expression in the mutants.
5) Mutant mice had D1 receptor binding and decreased NMDA receptor binding in the dentate gyrus compared to controls.
6) Of the genes researched, 6 were selectively expressed in the dentate gyrus according to the Allen Brain Atlas.
7) C-fos expression in knockouts was virtually wiped out compared to controls.
8) The number of BrdU-positive cells within the dentate gyrus was 52.8% higher (p = 0.00001) in the mutant mice than in the wild-type mice.
9) Mutant mice had fewer calbindin (mature neuron marker) labeled neurons, and an increased number of PSA-NCAM and calretnin (immature neuron markers) labeled neurons in the dentate gyrus compared to controls.
10) The neurons in the dentate gyrus of mutant mice had less branching and shorter dendrites than the neurons in control mice.
11) According to the electrophysiological recordings there was 1) increased input resistance in mutant mice (P = 0.0029), reduced spike latency in mutant mice (P < 0.0001), a smaller max number of spikes evoked by 400 ms depolarizing currents in mutant mice (P < 0.0001), and the efficacy of basal transmission at the mossy fiber synapse was increased in mutant mice (P < 0.0001). Also, there was reduced paired-pulse facilitation ratios of EPSPs at intervals ranging from 50 to 5000 ms in mutant mice.
12, A) The clustering of human brains according to expression of 10 genes that were differentially expressed in the mutant mice yielded 2 groups, one with higher incidence of schizophrenia (P = 0.0041).
12, B) There were 26 genes that had a greater than 2-fold highly significant difference in expression level, that were present in over 75% of hippocampi of at least one cluster group, and that were not affected by age or gender. Half of these are known to be involved in neurogenesis or cellular maturation.
- Summary
Alpha-CaMKII+/- mice had dysregulated behaviors including increased locomotor activity, a severe working memory deficit, and exaggerated infradian rhythm.
The mutants had abnormalities in gene expression and receptor binding in the hippocampus, specifically in the DG.
Molecular markers, morphological assessment, and electrophysiological analysis consistently showed that many of the neurons in the DG of the mutant mice failed to develop to maturity.
Transcriptome analysis of human post-mortem brain revealed biomarkers that could be used to identify people with higher susceptibility to schizophrenia.
These findings suggest that the "immature DG" or its equivalent is a brain phenotype that can reflect or affect susceptibility to psychiatric disorders.
- Discussion
Alpha-CaMKII may contribute to regulating gene expression required for the maturation of DG neurons via one of these three proposed pathways:
1) CaMKII-NeuroD pathway (CaMKII phosphorylates a transcription factor NeuroD at Ser336, stimulating dendritic growth).
2) Regulation of BDNF activation of dendritic growth (CamKII phosphorilates MeCP2 at Ser421, allowing MeCP2 to regulate BDNF).
3) BDNF-MAPK pathway (as evidenced by changes in gene expression of genes in pathway, mechanism not known).
Alpha-CaMKII may contribute to regulating gene expression of D1 receptors and NMDA receptors (via one of the 2000+ genes with differential gene expression) which could contribute to the electrophysiologogical differences seen in the mutants.
Alpha-CaMKII is expressed postnatally and dramatically increases from day 5 after birth, so postnatal alterations in DG maturation might trigger changes in intracortical connections, which may cause some of the behavioral phenotypes exhibited by the mutants.
Because knockouts were global, it isn’t known what brain regions contributed to behavioral, but dramatic changes in DG suggest that “immature DG” is at least involved in the behavioral abnormalities. DG is involved in locomotor activities, spatial working memory
The behavioral abnormalities in alpha-CaMKII+/- mice are similar to those observed in patients with psychiatric disorders; impairment in working memory is a proposed schizophrenia endophenotype, and exaggerated infradian rhythm is a prominent feature of bipolar disorder.
NMDA receptor is upstream of CaMKII and its decreased function is associated with schizophrenia, and may have a similar endophenotype to “immature DG.”
The post-mortem human tissue gene expression clustering study suggests that “immature DG” may be relatively common in schizophrenia, caused either by misexpression of CaMKII or other genes in the same pathway, so it’s a potential future endophenotype used for diagnosis or subgrouping of psychiatric patients.
MANUSCRIPT ID
- Title
Neural activity changes underlying the working memory deficit in alpha-camKii heterozygous mice
- Reference
Matsuo N, Yamasaki N, Ohira K, Takao K, Toyama K, Eguchi M, Yamaguchi S, Miyakawa T. Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front Behav Neurosci. 2009;3:20. Epub 2009 Sep 2.
- Abstract
The alpha-isoform of calcium/calmodulin-dependent protein kinase II (alpha-CaMKII) is expressed abundantly in the forebrain and is considered to have an essential role in synaptic plasticity and cognitive function. Previously, we reported that mice heterozygous for a null mutation of alpha-CaMKII (alpha-CaMKII+/-) have profoundly dysregulated behaviors including a severe working memory deficit, which is an endophenotype of schizophrenia and other psychiatric disorders. In addition, we found that almost all the neurons in the dentate gyrus (DG) of the mutant mice failed to mature at molecular, morphological and electrophysiological levels. In the present study, to identify the brain substrates of the working memory deficit in the mutant mice, we examined the expression of the immediate early genes (IEGs), c-Fos and Arc, in the brain after a working memory version of the eight-arm radial maze test. c-Fos expression was abolished almost completely in the DG and was reduced significantly in neurons in the CA1 and CA3 areas of the hippocampus, central amygdala, and medial prefrontal cortex (mPFC). However, c-Fos expression was intact in the entorhinal and visual cortices. Immunohistochemical studies using arc promoter driven dVenus transgenic mice demonstrated that arc gene activation after the working memory task occurred in mature, but not immature neurons in the DG of wild-type mice. These results suggest crucial insights for the neural circuits underlying spatial mnemonic processing during a working memory task and suggest the involvement of alpha-CaMKII in the proper maturation and integration of DG neurons into these circuits.
- Keywords
alpha-CaMKII, working memory, dentate gyrus, schizophrenia, immediate-early genes, c-fos
- Input Author
ACF
MANUSCRIPT DETAILS
- Introduction/Aims
α-CaMKII a protein kinase critical for LTP, learning, and memory: knock-out mice show deficits in both domains
Heterozygous K.O. mice have other phenotypes Behavioral : working memory deficits (proposed endophenotype of psychiatric disorders in humans), increased locomotor activity, decreased anxiety-like behavior, decreased depression-like behavior, periodic mood-change-like behavior Physiologic phenotypes: increased dopamine turnover in striatum and increased D1-like receptor binding in the dentate gyrus (DG) Cellular phenotypes: immature DG neurons (assayed by molecular profile, morphology, and electrophysiology
Immediate early genes (IEGs) are transcription factors whose expression has been used as a marker for neuronal activity as they are rapidly and transiently expressed following neuronal stimuli (examples are c-Fos and Arc)
The aim of the paper is to use IEG expression patterns following a working memory task to determine which brain regions are dysfunctional in α-CaMKII+/-
- Methods
Eight-arm radial maze (see figure)
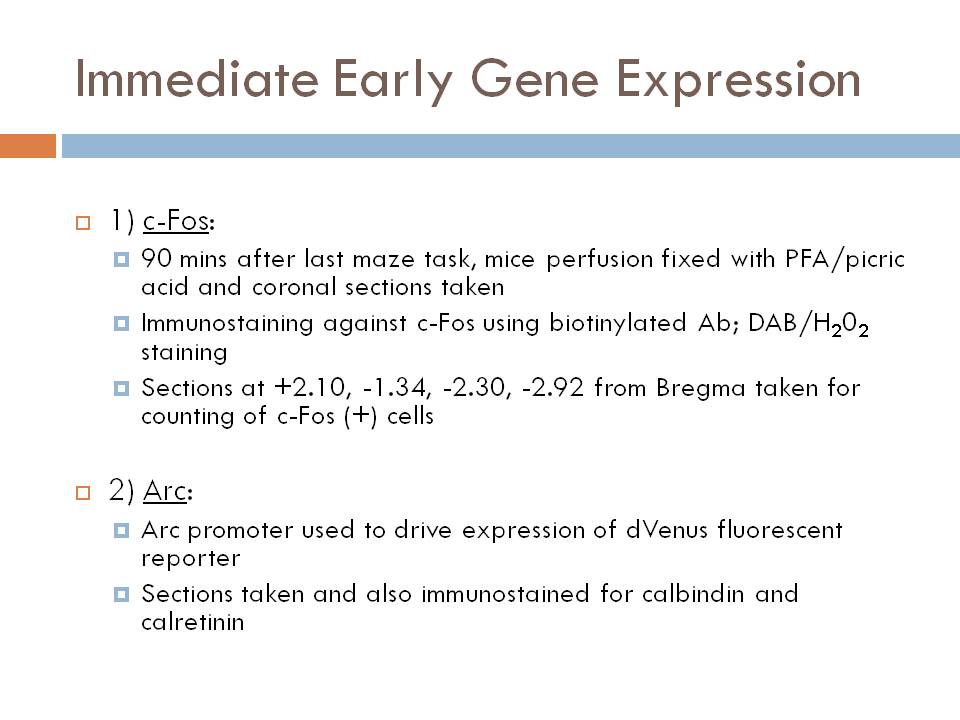
IEG expression (see figure)
- Results
Heterozygous CaMKII knockout mice have working memory deficits on the eight-arm radial maze test
c-Fos expression following the working memory test is reduced in K.O. animals in CA1, CA3, dentate gyrus (DG), central amygdaloid nucleus (CeA), and medial prefrontal cortex (mPFC)
Arc-dVenus expression was absent in the DG of K.O. animals after the working memory task
DG of K.O. was found to have reduced expression of calbindin (a marker of mature neurons) and increased expression of calretinin (a marker of immature neurons)
Little to no overlap occurred between cells active in the working memory task (Arc-dVenus positive) and immature neurons (calretinin positive); strong overlap occurred between mature neurons (calbindin positive) and active cells (Arc-dVenus positive)
- Discussion
DG function is implicated in working memory
Dysfunction in the DG likely underlies the deficits in hippocampal activity during the working memory task
The hippocampus projects to the mPFC and this connection is critical for working memory; dysfunction of the hippocampus likely underlies the deficits in mPFC activity during the working memory task
Aberrant growth and maturation of granule cells in the DG likely lead to deficits in DG activity during the working memory task