Difference between revisions of "Week9"
(→MANUSCRIPT Details) |
|||
| Line 1: | Line 1: | ||
| + | Back to '''[[NS210b | NS210b Class page]]''' | ||
| + | |||
== MANUSCRIPT ID == | == MANUSCRIPT ID == | ||
| − | |||
| − | |||
| − | * | + | *Title |
| − | * | + | *Reference |
| − | + | ||
| − | + | ||
| − | * | + | *Abstract |
| − | == MANUSCRIPT | + | *Keywords |
| + | |||
| + | *Input Author | ||
| + | |||
| + | |||
| + | |||
| + | == MANUSCRIPT DETAILS == | ||
*Introduction/Aims | *Introduction/Aims | ||
| − | + | *Methods | |
| − | + | ||
| + | *Results | ||
| + | |||
| + | *Summary | ||
| + | |||
| + | *Discussion | ||
| + | |||
| + | |||
| + | |||
| + | == MANUSCRIPT ID == | ||
| + | |||
| + | *Title | ||
| + | |||
| + | Learning to Control a Brain–Machine Interface for Reaching and Grasping by Primates | ||
| + | |||
| + | |||
| + | *Reference | ||
| + | |||
| + | Carmena JM, Lebedev Ma, Crist RE, et al. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS biology. 2003;1(2):E42. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14624244. | ||
| + | |||
| + | |||
| + | *Abstract | ||
| + | |||
| + | Reaching and grasping in primates depend on the coordination of neural activity in large frontoparietal ensembles. Here we demonstrate that primates can learn to reach and grasp virtual objects by controlling a robot arm through a closed-loop brain-machine interface (BMIc) that uses multiple mathematical models to extract several motor parameters (i.e., hand position, velocity, gripping force, and the EMGs of multiple arm muscles) from the electrical activity of frontoparietal neuronal ensembles. As single neurons typically contribute to the encoding of several motor parameters, we observed that high BMIc accuracy required recording from large neuronal ensembles. Continuous BMIc operation by monkeys led to significant improvements in both model predictions and behavioral performance. Using visual feedback, monkeys succeeded in producing robot reach-and-grasp movements even when their arms did not move. Learning to operate the BMIc was paralleled by functional reorganization in multiple cortical areas, suggesting that the dynamic properties of the BMIc were incorporated into motor and sensory cortical representations. | ||
| + | |||
| + | |||
| + | *Keywords | ||
| + | |||
| + | BMI, Brain-Machine Interface, Closed Loop, Directional Tuning, Primary Motor Cortex, Macaque, Linear Model, Robotics, Artificial Intelligence | ||
| + | |||
| + | |||
| + | *Input Author | ||
| + | |||
| + | JJM | ||
| + | |||
| + | |||
| + | == MANUSCRIPT DETAILS == | ||
| + | |||
| + | *Introduction/Aims | ||
| + | |||
| + | [[File:Example.jpg]] | ||
| + | |||
| + | Spinal cord injuries and neurodegeneration lead to thousands of motor deficits every year. Typically, research is aimed at reconstructing connectivity and function of damaged areas. This paper aims to bypass the damaged area entirely by recording from motor control areas and using those signals to control an external robotic prosthesis. | ||
| + | |||
| + | |||
| + | |||
| + | Primary motor cortex and surrounding areas probably control intentional motor movements, but there are many open questions regarding brain-machine interfaces recording from these areas. These questions include: | ||
| + | |||
| + | *What type of brain signal is the best input? Single unit? Multiunit? Local Field Potentials? | ||
| + | |||
| + | *Which cortical brain region(s) should we record from and contain the most information about motor movements? | ||
| + | |||
| + | *What types of motor commands can be extracted from cortical activity? | ||
| + | |||
| + | *How does an artifiicial actuator like a robot arm affect performance? | ||
| + | |||
| + | *How does use of a BMI reorganize cortical functionality? | ||
| + | |||
| + | This paper aims to explore these questions. | ||
| + | |||
| + | |||
| + | *Methods | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | The subjects in this study were two adult female macaque monkeys. Each monkey had training in interacting with a computer while in a chair. Recording arrays were implanted in primary motor cortex, dorsal premotor cortex, and supplementary motor cortex. Monkey 1 also had a recording array in primary sensory cortex. Monkey 2 also had a recording array in the medial intraparietal area of posterior parietal cortex. | ||
| + | |||
| + | [[File:Example.jpg]] | ||
| + | |||
| + | The experimental set-up was as follows. The monkeys were seated in a constrained chair facing a computer screen. In some trials the monkeys controlled a cursor on the screen by moving and squeezing a pole with their left arm. In other trials the pole was absent. Multiunit recording data from the abovementioned cortical areas was passed into a data acquisition box. A linear model was trained on the neural recording data to predict the position and squeeze strength of the cursor. In pole-control mode, the monkey directly controlled a robot arm; in brain-control mode, the linear model controlled the robot arm. The cursor's position on the screen was a reflection of the robot arm's position. | ||
| + | |||
| + | [[File:Example.jpg]] | ||
| + | |||
| + | In task 1, the monkey simply had to move the cursor to a target on the screen, and was given a juice reward upon successful completion in less than 5 seconds. | ||
| + | |||
| + | In task 2, the monkey had to apply the correct amount of force to expand the cursor to the proper size. | ||
| + | |||
| + | Task 3 was a combination of task 1 and 2. The monkey had to move the cursor to the target and then apply the correct pressure. | ||
| + | |||
| + | The linear model was a simple linear regression model, which finds the matrix of weights A that minimizes the mean squared error of the actual training outputs Y vs. X*A. Y is the output matrix over time, and X is the input matrix over time. A was found by the equation A = inv(X'X)X'Y, where inv(X) represents the inverse operation on a matrix, and X' represents the transpose operation on a matrix. The first 10 minutes of pole control activity was used as training, then the values of A were frozen in place for prediction. | ||
| + | |||
| + | |||
| + | *Results | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Summary | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *Discussion | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | *References | ||
Revision as of 18:06, 17 March 2010
Back to NS210b Class page
MANUSCRIPT ID
- Title
- Reference
- Abstract
- Keywords
- Input Author
MANUSCRIPT DETAILS
- Introduction/Aims
- Methods
- Results
- Summary
- Discussion
MANUSCRIPT ID
- Title
Learning to Control a Brain–Machine Interface for Reaching and Grasping by Primates
- Reference
Carmena JM, Lebedev Ma, Crist RE, et al. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS biology. 2003;1(2):E42. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14624244.
- Abstract
Reaching and grasping in primates depend on the coordination of neural activity in large frontoparietal ensembles. Here we demonstrate that primates can learn to reach and grasp virtual objects by controlling a robot arm through a closed-loop brain-machine interface (BMIc) that uses multiple mathematical models to extract several motor parameters (i.e., hand position, velocity, gripping force, and the EMGs of multiple arm muscles) from the electrical activity of frontoparietal neuronal ensembles. As single neurons typically contribute to the encoding of several motor parameters, we observed that high BMIc accuracy required recording from large neuronal ensembles. Continuous BMIc operation by monkeys led to significant improvements in both model predictions and behavioral performance. Using visual feedback, monkeys succeeded in producing robot reach-and-grasp movements even when their arms did not move. Learning to operate the BMIc was paralleled by functional reorganization in multiple cortical areas, suggesting that the dynamic properties of the BMIc were incorporated into motor and sensory cortical representations.
- Keywords
BMI, Brain-Machine Interface, Closed Loop, Directional Tuning, Primary Motor Cortex, Macaque, Linear Model, Robotics, Artificial Intelligence
- Input Author
JJM
MANUSCRIPT DETAILS
- Introduction/Aims
Spinal cord injuries and neurodegeneration lead to thousands of motor deficits every year. Typically, research is aimed at reconstructing connectivity and function of damaged areas. This paper aims to bypass the damaged area entirely by recording from motor control areas and using those signals to control an external robotic prosthesis.
Primary motor cortex and surrounding areas probably control intentional motor movements, but there are many open questions regarding brain-machine interfaces recording from these areas. These questions include:
*What type of brain signal is the best input? Single unit? Multiunit? Local Field Potentials?
*Which cortical brain region(s) should we record from and contain the most information about motor movements?
*What types of motor commands can be extracted from cortical activity?
*How does an artifiicial actuator like a robot arm affect performance?
*How does use of a BMI reorganize cortical functionality?
This paper aims to explore these questions.
- Methods
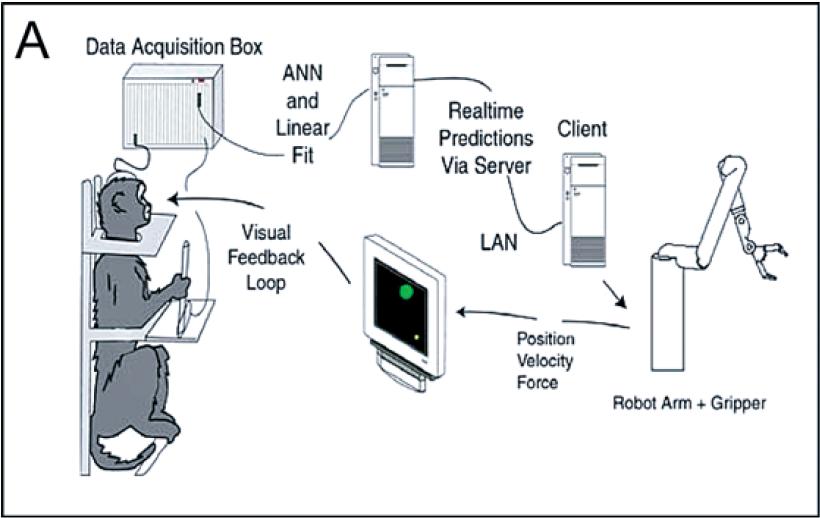
The subjects in this study were two adult female macaque monkeys. Each monkey had training in interacting with a computer while in a chair. Recording arrays were implanted in primary motor cortex, dorsal premotor cortex, and supplementary motor cortex. Monkey 1 also had a recording array in primary sensory cortex. Monkey 2 also had a recording array in the medial intraparietal area of posterior parietal cortex.
The experimental set-up was as follows. The monkeys were seated in a constrained chair facing a computer screen. In some trials the monkeys controlled a cursor on the screen by moving and squeezing a pole with their left arm. In other trials the pole was absent. Multiunit recording data from the abovementioned cortical areas was passed into a data acquisition box. A linear model was trained on the neural recording data to predict the position and squeeze strength of the cursor. In pole-control mode, the monkey directly controlled a robot arm; in brain-control mode, the linear model controlled the robot arm. The cursor's position on the screen was a reflection of the robot arm's position.
In task 1, the monkey simply had to move the cursor to a target on the screen, and was given a juice reward upon successful completion in less than 5 seconds.
In task 2, the monkey had to apply the correct amount of force to expand the cursor to the proper size.
Task 3 was a combination of task 1 and 2. The monkey had to move the cursor to the target and then apply the correct pressure.
The linear model was a simple linear regression model, which finds the matrix of weights A that minimizes the mean squared error of the actual training outputs Y vs. X*A. Y is the output matrix over time, and X is the input matrix over time. A was found by the equation A = inv(X'X)X'Y, where inv(X) represents the inverse operation on a matrix, and X' represents the transpose operation on a matrix. The first 10 minutes of pole control activity was used as training, then the values of A were frozen in place for prediction.
- Results
- Summary
- Discussion
- References